IT solutions
Successfully Transfer Robust Methods between Labs Using Method Development Software
Jun 04 2024
Chromatographic methods are crucial for separating and analyzing complex mixtures—a process essential for research and development. Robust chromatographic methods are developed so that small variations in conditions do not impact critical attributes which is often laborious and time-consuming.
Analytical laboratories do not work in isolation, there is increasing collaboration across partners (i.e. CxOs) and facilities with methods being transferred between them. When transferring methods, they are adapted to different chromatographic systems. Differences in hardware (columns, detectors, chromatographic systems) and software capabilities, especially between different instrument vendors, cause variability in method performance.
For methods to be successfully transferred between labs, the method must perform consistently with replicable results across various setups. Tools within ACD/Labs’ Method Selection Suite software help streamline the transfer of robust, reproducible methods without impacting method performance.
Automatically Generate Methods for All Possible Combinations of Variations in Method Parameters
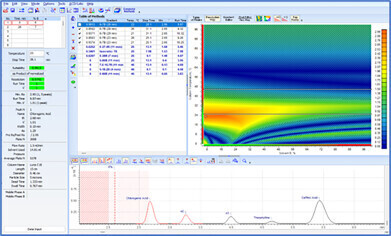
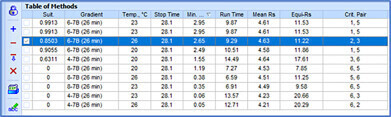
Before an analytical method can be transferred, it must be evaluated for variations in method parameters and the impact on chromatographic separation must be understood. Method Selection Suite streamlines the process, minimizing manual input. With just a few clicks, the vary method parameters feature adjusts the parameters of a developed method by specified amounts (either one at a time or simultaneously), and automatically generates methods for all possible combinations of parameter variations. The resulting chromatograms can highlight the maximum anticipated variation based on specific method parameters and conditions, alerting scientists of potential risks.
Ensure Quality, Robustness, and Reproducibility
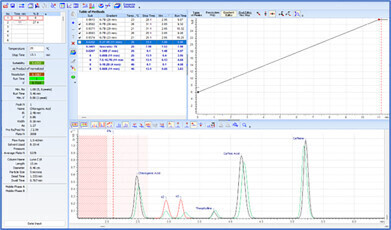
To maintain proper control over methods, it is crucial to identify critical parameters that significantly impact chromatographic output (i.e., peak shift, resolution, etc.). Using the vary method parameters feature, chromatographers can assess how method parameters affect the resulting chromatogram. This enables them to determine whether anomalies between transferred methods stem from variations in critical parameters prompting corrective action, or from factors beyond their control, thereby avoiding unnecessary experimentation. Understanding the influence of parameters on method quality is essential for saving time and preventing method failure during transfer.
Deeper insight into the method development process is achieved through resolution maps and the visualization of chromatograms. The center point on resolution maps helps to identify optimal separation conditions and ensure maximal method robustness. Overlaying experimental and predicted methods allows scientists to quickly observe variations, pinpointing the specific parameter (or combination of parameters) responsible—ensuring specific problems are addressed and/or appropriate control measures are implemented.
Integrate Data Management for Easy Data Accessibility and Collaboration
Using Method Selection Suite all chromatograms, methods (original and varied parameters), metadata, structures, retention time, etc., are added to the easily searchable and accessible database, making it easy to access and share knowledge among internal and external partners.
Method Selection Suite allows scientists to optimize and control parameters, ensuring methods are transferred without impacting performance. The feature to vary method parameters allows scientists to predict chromatographic behavior within specified bounds—ensuring the replication of methods between labs while maintaining quality and control.
Digital Edition
Lab Asia 32.2 April
April 2025
Chromatography Articles - Effects of small deviations in flow rate on GPC/SEC results Mass Spectrometry & Spectroscopy Articles - Waiting for the present to catch up to the future: A bette...
View all digital editions
Events
Apr 22 2025 Hammamet, Tunisia
Apr 22 2025 Kintex, South Korea
Analytica Anacon India & IndiaLabExpo
Apr 23 2025 Mumbai, India
Apr 23 2025 Moscow, Russia
Apr 24 2025 Istanbul, Turkey