Chromatography
Transmission Raman Probes API Content Uniformity of Pharmaceutical Tablets
Jun 07 2010
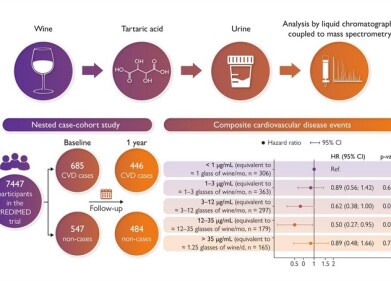
This new application note describes the use of Transmission Raman spectroscopy for the determination of content uniformity in pharmaceutical tablets, not only for in initial formulation protocol development, but as a routinely usable PAT at-line method for QC.
The fact that conventional Raman only examines the surface, not the entire volume, has long prevented Raman spectroscopy from being considered as a serious candidate for content uniformity assessment. The new transmission-mode measurement geometry addresses this issue. This new sampling approach consists in exciting from one side of the sample while collecting the signal from the other side and hence provides the ability of NIR to examine a large volume of the sample with the specificity of Raman to yield excellent correlation with the current off-line destructive method. Moreover, the measurement can be completed in a few seconds or less.
For a free copy of the application note please contact us at info-sci.fr@horiba.com or come to see us at www.horiba.com/scientific
Digital Edition
Lab Asia 31.6 Dec 2024
December 2024
Chromatography Articles - Sustainable chromatography: Embracing software for greener methods Mass Spectrometry & Spectroscopy Articles - Solving industry challenges for phosphorus containi...
View all digital editions
Events
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK
Jan 25 2025 San Diego, CA, USA
Jan 27 2025 Dubai, UAE
Jan 29 2025 Tokyo, Japan