-
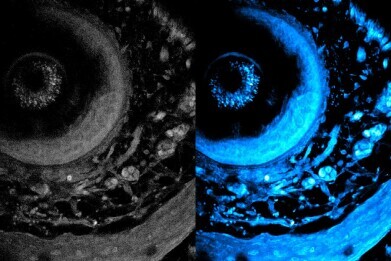 On left is the initial image, and on right is the optimised image using the new technique. The technique enables laser light to penetrate deeper into living tissue, which captures sharper images of cells at different layers of a living system. Picture credit: MIT researchers
On left is the initial image, and on right is the optimised image using the new technique. The technique enables laser light to penetrate deeper into living tissue, which captures sharper images of cells at different layers of a living system. Picture credit: MIT researchers
Research News
MIT non-invasive imaging method uses high-powered lasers to penetrate ever deeper into living tissue
Dec 31 2024
Method will allow study of the body’s immune response
Metabolic imaging is a non-invasive method that enables clinicians and scientists to study living cells using laser light, which can help them assess disease progression and treatment responses.
However, light scatters when it is shone into biological tissue, consequently limiting the depth to which it can penetrate. This has limited the resolution of captured images.
Researchers at the Massachusetts Institute of Technology (MIT) have developed a technique that more than doubles the previous depth limit from standard of care metabolic imaging. Imaging speeds have also been boosted producing richer and more detailed images.
The novel technique uses a specialised laser that illuminates deep into the tissue, which causes certain intrinsic molecules within the cells and tissue to emit light. This eliminates the need to alter the tissue ─ by cutting it or staining it with dyes ─ and so provides a more accurate representation of its structures and functions.
Greater penetration depth, faster speeds, and higher resolution make this method particularly well-suited for demanding imaging applications like cancer research, tissue engineering, drug discovery, and the study of immune responses.
The MIT laser can customise the light emitted for examination of deep tissues. A ‘fibre shaper’ the researchers developed ─ a device controlled by bending it ─ it can be tuned by colour and light pulse to minimise scattering and maximise signal, as the light travels deeper into the tissue. Particularly with living tissue, this will allow the clinician to see much further into the tissue and capture clearer images.
“This work shows a significant improvement in terms of depth penetration for label-free metabolic imaging. It opens new avenues for studying and exploring metabolic dynamics deep in living biosystems,” says Sixian You, assistant professor in the Department of Electrical Engineering and Computer Science (EECS), a member of the Research Laboratory for Electronics, and senior author of a paper on this imaging technique.
She is joined on the paper by lead author Kunzan Liu, an EECS graduate student; Tong Qiu, an MIT postdoc; Honghao Cao, an EECS graduate student; Fan Wang, professor of brain and cognitive sciences; Roger Kamm, the Cecil and Ida Green Distinguished Professor of Biological and Mechanical Engineering; Linda Griffith, the School of Engineering Professor of Teaching Innovation in the Department of Biological Engineering; and other MIT colleagues. The research has been published in Science Advances.
The method falls in the category of ‘label-free’ imaging, meaning tissue is not stained beforehand. Staining creates contrast that helps a clinical biologist see cell nuclei and proteins better. But it typically requires the sample to be sectioned and sliced in the laboratory, which often kills the tissue makes it impossible to study dynamic processes in living cells.
In label-free imaging techniques, researchers use lasers to illuminate specific molecules within cells, causing them to emit different coloured light of that reveal various molecular contents and cellular structures. However, generating the ideal laser light with certain wavelengths and high-quality pulses for deep-tissue imaging has been the researcher’s challenge.
Building on prior work, the researchers adapted the first version of the fibre shaper for deeper multimodal metabolic imaging. Once they had built the controllable mechanism, they developed an imaging platform to leverage the powerful laser source to generate longer wavelengths of light, which are crucial for deeper penetration into biological tissues.
When the imaging device was tested, the light it emitted was able to penetrate more than 700 micrometres into a biological sample. The previous best techniques could only reach around 200 micrometres.
This new imaging method could offer a boost to the study of organoids, which are engineered cells that can grow to mimic the structure and function of organs.
With these and other biomedical applications in mind, the researchers plan to aim for even higher-resolution images. At the same time, they are working to create low-noise laser sources, which could enable deeper imaging with less light dosage.
They are also developing algorithms that react to the images to reconstruct the full 3D structures of biological samples in high resolution.
In the long run, they hope to apply this technique in the real world to help biologists monitor drug response in real-time to aid in the development of new medicines.
The research was funded, in part, by MIT startup funds, a US National Science Foundation CAREER Award, an MIT Irwin Jacobs and Joan Klein Presidential Fellowship, and an MIT Kailath Fellowship.
Digital Edition
Lab Asia 31.6 Dec 2024
December 2024
Chromatography Articles - Sustainable chromatography: Embracing software for greener methods Mass Spectrometry & Spectroscopy Articles - Solving industry challenges for phosphorus containi...
View all digital editions
Events
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK
Jan 25 2025 San Diego, CA, USA
Jan 27 2025 Dubai, UAE
Jan 29 2025 Tokyo, Japan