Laboratory Products
Automated Particle Imaging System used to troubleshoot Particle Sizing Methods
Nov 01 2013
Scientists at Reading Scientific Services Ltd which provides analysis, research, consultancy and training services for the food, pharmaceutical and healthcare industries, are using a Morphologi G3 automated particle imaging system from Malvern Instruments to troubleshoot particle sizing methods for pharmaceutical clients and develop fully validated procedures.
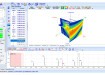
The Morphologi G3 delivers statistically relevant particle size and shape data that enable the investigation of factors that can have an effect on particle size measurement, such as dispersion state and crystal shape. Running automated particle imaging alongside laser diffraction particle sizing using the Mastersizer 2000 supports the development of truly robust test methods that may be applied to characterise products from developmental through to commercial scale manufacture.
“RSSL develops particle size analysis methods for pharmaceutical clients that are fully validated in line with regulatory requirements,” explained Chris Aiken, Physical Sciences Laboratory Manager, RSSL. “To do this we need to ensure that the method we provide gives tight control over any source of potential variability. Sometimes particle shape measurements may be used to troubleshoot suspected dispersion issues that can be encountered during laser diffraction measurement. In a recent project, for example, the images from the Morphologi G3 provided compelling evidence that the particles, rather than the sizing method, were a root cause of observed variability. Here the imaging results helped us to modify the existing particle sizing method so that it would work successfully for the commercially manufactured product.”
In a recent study, a pharmaceutical manufacturer approached RSSL with a size measurement issue that had arisen following scale-up of the process to commercial manufacture of an active pharmaceutical ingredient (API). Automated image analysis demonstrated that in the small/pilot scale manufacturing process, API crystal shape was consistent. However, during commercial API manufacturing processes either cubic or rod-shaped crystals were produced. Consequently, variability was observed in the particle size results returned by the laser diffraction measurement method for the commercial scale batch. By intelligently modifying the laser diffraction method to tackle the issue, RSSL delivered an appropriate, fully validated technique for monitoring the manufacturing process.
Digital Edition
International Labmate 49.6 - Sept 2024
September 2024
Chromatography Articles - HPLC gradient validation using non-invasive flowmeters Mass Spectrometry & Spectroscopy Articles - From R&D to QC, making NMR accessible for everyone: Putting NMR...
View all digital editions
Events
Oct 06 2024 Liverpool, UK
Oct 08 2024 Gothenburg, Sweden
Oct 09 2024 Birmingham, UK
Oct 09 2024 NEC, Birmingham, UK
Oct 15 2024 Milan, Italy