Laboratory Products
Nanoparticle Tracking Analysis Used to Characterise Novel Nanoscale Reference Materials
Oct 05 2012
LGC is the UK's designated National Measurement Institute for chemical and bioanalytical measurement, the National Reference Laboratory for a range of key areas, and is also the host organisation for the UK's Government Chemist function.
LGC is developing a standardised panel of reference nanomaterials to enable the development of traceable methods for improved in vitro toxicity measurement for safety assessment.
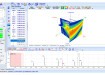
Potential toxicity is difficult to predict. To address this, LGC is developing a panel of nanoparticle reference materials which will be assessed for their physical and chemical properties in an in vitro biological matrix. These reference materials will be based on the high quality nanoparticles produced by the EU-Joint Research Centre which have undergone extensive physical characterisation in the dry-powder form. LGC are carrying out the characterisation work using a unique combination of Nanoparticle Tracking Analysis (NTA) from NanoSight, which images and tracks the Brownian motion of particles in solution, with field flow fractionation coupled to inductively coupled plasma mass spectrometry. This approach will allow complex suspensions of nanomaterials to be characterised for their size, size distribution, charge, concentration, dissolution and elemental composition. Furthermore, NTA is now recognised by ASTM E2834 as a standard for the Measurement of Particle Size Distribution of Nanomaterials in Suspension. With its unique particle by particle method of measurement, NTA provides the ideal insight for this characterisation challenge.
Speaking about this project, Damian Marshall said "It is anticipated that this research will produce a prototype panel of reference materials characterised for their properties in physiologically relevant systems so they can be applied as calibration standards in routine testing procedures. The research should support public acceptance of nanomaterial safety by providing reference materials that can be incorporated into testing regimes for regulatory processes."
Concluding, Marshall stated "Working together with NanoSight's technical team and its development of NTA has enabled us to significantly improve our toxicity measurements. The efficient sampling process means we are now able to measure size and charge of nanoparticles in cell culture media over a given time period. This has been invaluable in assisting our understanding of how nanoparticles behave in biological systems."
Digital Edition
International Labmate 49.6 - Sept 2024
September 2024
Chromatography Articles - HPLC gradient validation using non-invasive flowmeters Mass Spectrometry & Spectroscopy Articles - From R&D to QC, making NMR accessible for everyone: Putting NMR...
View all digital editions
Events
Sep 29 2024 Singapore
Oct 06 2024 Liverpool, UK
Oct 08 2024 Gothenburg, Sweden
Oct 09 2024 Birmingham, UK
Oct 09 2024 NEC, Birmingham, UK



.jpg)