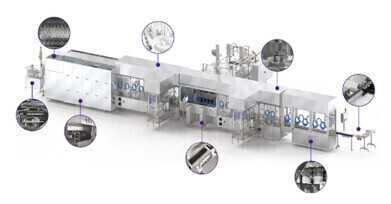
Laboratory products
Cutting-edge solutions for precision and sterility in aseptic fill-finish
Feb 12 2024
Increased demand for injectable drugs and stricter regulations drive surge in aseptic fill-finish technology, with Biopharma Group emerging as a trusted partner for UK and Irish pharma companies.
Why is aseptic fill-finish critical? This process safeguards the efficacy, integrity, and ultimately, the safety of sterile medications. With the UK and Ireland's aseptic fill-finish market projected to reach £3.8 billion by 2025, Biopharma Group highlights the importance of:
- Precision: Choosing the right equipment for accurate dosing and sterility is paramount
- Compliance: Modern systems prioritise features like validated cleaning, data integrity, and container closure integrity testing
- Patient Safety: Integrated cleanroom environments, advanced sterilisation technologies, and automated inspection systems minimise contamination risks
Case Study: A leading pharmaceutical company reduced waste, increased efficiency, and improved product quality with Biopharma Group's aseptic fill-finish solutions. The results were very impressive:
- 50% reduction in waste product
- 20% increase in production efficiency
- 30% reduction in downtime
- Significant improvement in product quality observed
- Clear return on investment
Biopharma Group's Value Proposition:
- Partnerships: Collaborating with ATS Scientific Life Sciences, Biopharma Group offers a comprehensive equipment range
- Expertise: 35 years of experience in the UK and Irish markets
- Solutions:
- Aseptic fill-finish equipment: Isolators, RABS, filling lines, inspection systems
- Freeze dryers and lyo services: Expertise in lyophilisation with advanced freeze dryers and validated processes
- Technical support and training: Comprehensive support throughout the process
Elevate your injectable drug production with Biopharma Group's commitment to precision and sterility in aseptic fill-finish.
Contact Biopharma Group today to discuss your requirements.
More information online
Digital Edition
Lab Asia 31.6 Dec 2024
December 2024
Chromatography Articles - Sustainable chromatography: Embracing software for greener methods Mass Spectrometry & Spectroscopy Articles - Solving industry challenges for phosphorus containi...
View all digital editions
Events
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK
Jan 25 2025 San Diego, CA, USA
Jan 27 2025 Dubai, UAE
Jan 29 2025 Tokyo, Japan