Laboratory Products
Microwave Irradiation and Small-Angle X-ray Scattering: A Powerful Combination for the Preparation and Characterisation of Nanomaterials
Jul 09 2013
Author: A. Stadler, A. Keilbach, H. Santner on behalf of Anton Paar GmbH
Microwave-assisted synthesis was originally developed to enhance standard processes in organic chemistry and has been successfully used in numerous chemistry disciplines for more than two decades. In recent years this technology has also attracted considerable interest in applications related to inorganic chemistry. This non-classical heating source became especially popular in the synthesis of nanomaterials due to its ability to drastically reduce reaction times.
Small-angle scattering, SAXS for short, is a valuable method for the analysis of nanomaterials. SAXS provides information on the size and structure of nano-sized materials in the range from typically 1nm to 200nm. The results obtained by SAXS are representative for an entire sample and therefore provide important information on the nanomaterial to complement the information obtained by e.g. electron microscopy, which only produces local, non-representative information on a small portion of the sample. SAXS is a non-destructive method and typically requires no sample preparation.
The usually rather time-consuming hydrothermal or solvothermal processes under conventional heating can be significantly enhanced by rapid heating of the reaction mixtures far beyond their boiling point. Furthermore, the exact determination of relevant reaction parameters such as temperature and pressure in combination with short reaction times allows tailoring the desired nanomaterials to achieve various physical and chemical properties. Among the most attractive nano compounds in current research are several metal oxides and phosphates, which are of interest for efficient energy storage systems, as well as selenium containing quantum dots for manufacturing nanocomposites used in light-emitting diodes or solar cells.
As evidenced in recent research, the beneficial effect of microwave irradiation in general and in the synthesis of nanomaterials in particular can be simply attributed to thermal effects due to higher reaction temperatures reached in a shorter time [1-3].
Since typically the reaction temperatures employed are far beyond the boiling point of the used solvent, the accurate measurement and control of this relevant reaction parameter is required. Modern microwave instruments usually provide a reliable IR sensor for temperature control, whereas Anton Paar’s Monowave 300 additionally offers an immersing fibre-optic sensor for accurate and immediate sensing of the internal reaction temperature. This immediate in-situ determination of the key reaction parameter leads to a rapid optimisation of the reaction process and provides an easy way to tailor the structure and morphology of the desired nanomaterials. Kappe and co-workers evaluated the hydrothermal synthesis of ZnO nanorods and found the exact achievement of the optimum reaction temperature of 150°C crucial since any higher reaction temperature has a negative effect on the product quality [2]. At 150°C the growth of the nanorods can be nicely controlled by the irradiation time from 1 to 10 minutes leading to continuously longer rods while the diameter remains constant.
In a related work Kappe’s group optimised the microwave-assisted synthesis of industrially attractive CdSe quantum dots and combined it with size distribution monitoring by small-angle X-ray scattering (SAXS) [3]. This ground-breaking investigation showed clearly that the electromagnetic field itself has no impact on the tunable synthesis of such monodisperse colloidal nanocrystals.
As proven by the subsequent SAXS experiments the morphology of the resulting quantum dots can be influenced by the employed cadmium sources, the hold time at the target temperature as well as the duration of the injection of the stabilising agent.
In the corresponding example the best results for the microwave-assisted synthesis were achieved at 240°C within an overall process time of less than 20 minutes, which is about only one quarter of the reaction time required under conventional heating conditions [2].
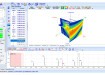
Small-angle X-ray scattering was used to study the effects of varying synthesis conditions on the size and size distribution of the obtained quantum dot dispersions. The quantum dot samples, dispersed in Heptane, were filled into a quartz capillary sample holder and were analysed using Anton Paar's laboratory SAXS system equipped with a sealed X-ray tube source (2 kW) and a one-dimensional silicon diode array detector. Exposure time was varied between 1 and 5 minutes. The solvent Heptane was measured under identical conditions for background subtraction.
The obtained scattering patterns were processed in the SAXSquant program, the pair-distance distribution functions and size distributions were calculated with the GIFT program [4]. The obtained results quickly showed the impact of the synthesis conditions on the size of the quantum dots as well as if they are mono- or polydisperse. The size distribution of quantum dots synthesised under optimised conditions is shown in Figure 3. It indicates highly monodisperse nanoparticles with an average radius of approx. 1.7nm.
In summary it could be shown that the combination of two effective, rapid technologies leads to a significant improvement and speeding up of development and optimisation of nanomaterial synthesis. Microwave irradiation allows for short synthesis protocols in general and SAXS is a perfect tool to optimise these synthesis processes since it quickly and precisely determines particle size and size distribution of intermediates and final products during all stages of the synthesis. Thus, within a few short time experiments the optimum protocols to beneficially control size and size distribution of the quantum dots and therefore tailoring its electronic structure could be elaborated.
Finally, due to the accurate internal temperature measurement, the optimised microwave protocols can be easily transferred from Monowave 300 to scale-up devices like Anton Paar’s Masterwave BTR to quickly synthesise significant gram amounts of valuable nanomaterials. Wherever time equals money microwave-assisted synthesis is a beneficial way to prepare industrially relevant compounds.
References
[1] D. Obermayer, B. Gutmann, C.O. Kappe, Angew. Chem. Int. Ed . 2009, 48, 8321
[2] M. Baghbanzadeh, S. D. Skapin, Z. C. Orel, C. O. Kappe, Chem. Eur. J. 2012, 18, 5724
[3] M. M. Moghaddam, M. Baghbanzadeh, A. Keilbach, C. O. Kappe, Nanoscale, 2012, 4, 7435
[4] G. Fritz, O. Glatter, J. Phys.: Condens. Mat. 2006,
Digital Edition
International Labmate 49.6 - Sept 2024
September 2024
Chromatography Articles - HPLC gradient validation using non-invasive flowmeters Mass Spectrometry & Spectroscopy Articles - From R&D to QC, making NMR accessible for everyone: Putting NMR...
View all digital editions
Events
Oct 06 2024 Liverpool, UK
Oct 08 2024 Gothenburg, Sweden
Oct 09 2024 Birmingham, UK
Oct 09 2024 NEC, Birmingham, UK
Oct 15 2024 Milan, Italy