Analytical instrumentation
How HPLC and online LC technology is helping to create a new fuel industry out of thin air
Dec 23 2024
Author: Dr. Alessandro Senocrate on behalf of Agilent Technologies
Free to read
Articles are free to download. Unlock the article to be shown more content, graphs and images.
Our fossil fuel-driven economy currently releases around 40 gigatonnes of carbon dioxide (CO2) into the atmosphere every year, a number that continues to rise. Natural sinks like oceans and plants currently absorb about half of this CO2, however not without significant environmental issues. The remaining half stays in the atmosphere, exacerbating climate change.
However, this excess CO2 holds untapped potential as a resource for the production of platform chemicals and synthetic fuels, known as synfuels. These drop-in alternatives to fossil fuels have a range of applications and are especially useful in sectors that require high energy density options and are very difficult to electrify, such as road haulage, aviation, and marine transport.
At Empa, the Swiss Federal Laboratories for Materials Science and Technology, we envision a future where we will be able to remove CO2 from the atmosphere – or avoid its emission by capturing it at the source, such as at industrial sites – and to repurpose it as a valuable feedstock. Funded by a Swiss National Science Foundation (SNSF) Ambizione grant, by the SNSF National Competence Center for Research in Catalysis (NCCR Catalysis) and by the Swiss Federal Office of Energy (SFOE) through the SWEET project refuel.ch, our research focuses on electrochemically converting CO2 into sustainable fuels or platform chemicals. This conversion takes place in electrochemical cells powered by renewable electricity and utilizes an aqueous electrolyte, that is water containing dissolved salts. Despite its promise, and the fast progress taking place in the field, this conversion process is yet to be fully understood and remains challenging to implement on a large scale.
Measuring outputs
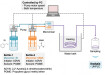
A copper catalyst – the material most used for CO2 conversion – produces around 15 to 20 different types of molecules during the electrochemical conversion of CO2. These include hydrocarbons such as methane (CH4) and ethylene (C2H4), as well as alcohols like methanol (CH3OH) and ethanol (C2H5OH), which can be used as fuels or as building blocks for other chemicals. From a practical point of view, separating these products can be difficult, energy intensive, and costly, as some are liquid at room temperature, others are gaseous, and some are in ionic form inside the electrolyte. Therefore, a key part of our research is to understand which parameters drive a catalyst’s product selectivity, with the final goal to obtain a single desired product, or an easily separable mixture. To do so, we must monitor the product outputs during all experiments, and we need to be able to accurately and rapidly capture both high and low analyte concentrations, as certain products are present only in parts per million but are still relevant for our understanding of the CO2 conversion reaction.
To monitor the gas and liquid product outputs, we use gas chromatography, which provides results within about three minutes, and we employ Agilent’s online high-performance liquid chromatography (HPLC) system, which allows us to continuously analyze our electrolytes by automatically extracting samples and either injecting them into the HPLC or storing them in sealed vials.
These methods are crucial as they enable us to track production over time, not just at the end of the process, without the need for constant manual intervention. Regularly measuring outputs while controlling variables such as voltage and pressure within the cells is essential for obtaining reproducible results. By integrating our own open-source analytical software, we have also streamlined the analysis process, reducing the time required to evaluate a dataset from hours to minutes.
The power of partnerships
The collaboration with Agilent began with the suggestion that we try high-performance liquid chromatography (HPLC) to address these analytical challenges, traditionally seen as more suited for gas chromatography. The HPLC implementation showed immediate promise but required manual labor to extract liquid samples during CO2 electrocatalysis. Fortunately, this sampling challenge was also an ideal match for the then-unreleased online liquid chromatography system. Empa was the first institute to use this online system. This ongoing collaboration has been instrumental in advancing research and scaling up technologies for industrial application, demonstrating the significant impact of strategic partnerships in driving innovation and achieving scientific breakthroughs.
A catalyst for change
The research at Empa aims at developing not only advanced methodologies for electrochemical CO2 conversion, but also novel electrode architectures and tailored catalysts. To give just one example, our SNSF Ambizione project focuses on tailoring catalytic properties using so-called defects, errors in a crystalline material caused by missing or replaced atoms in its lattice. These defects introduce unique properties into their host materials, which, during electrochemical CO2 reduction, can influence which molecules are produced. The vision is that after understanding the defect properties, we will be able to design tailored catalytic materials able to produce specific sustainable fuels or chemicals. This and other approaches bring us closer to delivering scalable CO2 conversion technologies for a greener and more efficient future.
Across the globe, many research efforts targeting CO2 electrolysis are underway. For example, the European Union has funded several initiatives, including the CO2-based electrosynthesis of ethylene oxide (CEO) project. Meanwhile, the Massachusetts Institute of Technology (MIT) is developing carbon nanotube membranes to enhance the efficiency of CO2 capture. Although there is unlikely to be a single solution to the CO2 problem, Empa’s work demonstrates considerable promise.
The research in electrochemical CO2 conversion is also part of a broader network of emerging technologies aimed at environmental protection and the development of a green economy. Our collaborations with technology partners like Agilent play a crucial role in advancing research and scaling up technologies for industrial applications. These partnerships are essential for moving CO2 conversion technologies from the lab to the market, where they can have a tangible impact.
Economic and environmental benefits
CO2-based fuels and chemicals have an important role to play in building a sustainable economy. The work at Empa, and the support by its technology partners, has the potential for delivering these benefits while also creating jobs, driving economic growth, and fostering technological innovation. The most immediate and significant advantage, however, is the reduction of greenhouse gas emissions.
By capturing CO2 and using it to produce sustainable fuels and chemicals, we prevent its release into the atmosphere, thereby mitigating its contribution to global warming. Moreover, reducing our reliance on fossil fuels helps to lessen the environmental impact associated with their extraction and production, including air and water pollution, habitat destruction, and oil spills. Additionally, some methods of sustainable fuel and chemical production using captured CO2 could become more energy-efficient than traditional processes, leading to lower overall energy use and reduced waste.
However, none of this would be possible without ongoing innovation and cooperation. As we continue to explore the potential of CO2 conversion, our efforts contribute to the broader goal of building a sustainable, low-carbon economy.
Bibliography
Senocrate, A.; Bernasconi, F.; Kraus, P.; Plainpan, N.; Trafkowski, J.; Tolle, F.; Weber, T.; Sauter, U.; Battaglia, C. Parallel Experiments in Electrochemical CO2 Reduction Enabled by Standardized Analytics. Nat Catal 2024, 7 (6), 742–752. https://doi.org/10.1038/s41929-024-01172-x.
Dr. Alessandro Senocrate is an SNSF Ambizione group leader at Empa, the Swiss Federal Laboratories for Materials Science and Technology. With a strong background in electrochemistry and materials science, Dr. Senocrate researches energy conversion and storage technologies, particularly in the field of electrochemical conversion of CO2 into sustainable fuels and chemicals. His research aims at understanding and improving the efficiency and sustainability of these energy storage systems. Dr. Senocrate has contributed significantly to the scientific community through numerous publications and collaborations.
Free to read
Articles are free to download. Please login to read this article or create an account.
Digital Edition
Lab Asia 31.6 Dec 2024
December 2024
Chromatography Articles - Sustainable chromatography: Embracing software for greener methods Mass Spectrometry & Spectroscopy Articles - Solving industry challenges for phosphorus containi...
View all digital editions
Events
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK
Jan 25 2025 San Diego, CA, USA
Jan 27 2025 Dubai, UAE
Jan 29 2025 Tokyo, Japan