Laboratory Products
Agreement Secured to Develop Assays to Assist Pre-Eclampsia Diagnosis
Dec 06 2011
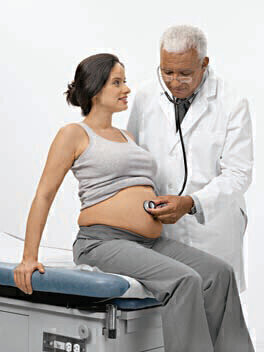
Siemens Healthcare Diagnostics has entered into a global licensing agreement with Nephromics LLC to develop two assays to be used as an aid in the diagnosis of pre-eclampsia. The condition is potentially life threatening and occurs during pregnancy affecting both mothers and their unborn children. The assays are being developed for the detection of two biomarkers, soluble fms-like tyrosine-kinase-1 (SFLT-1) and placental growth factor (PLGF), which clinical studies have shown to provide early identification of patients with pre-eclampsia. “Given the prevalence and associated mortality with preeclampsia, clinicians need better diagnostic tools to identify the condition before it becomes advanced,” said Dave Hickey, CEO, Chemistry, Immunoassay, Automation, and Diagnostics IT Business Unit at Siemens Healthcare Diagnostics. “The development of assays designed to detect pre-eclampsia earlier is an evolving area of diagnostic medicine - we are excited to enter into the agreement with Nephromics to offer these diagnostic solutions to our customers, clinicians and patients.”
Under the terms of the agreement with Nephromics, Siemens Healthcare Diagnostics will obtain rights for the development of the SFLT-1 and PLFG assays to be used in conjunction with each other to aid in the diagnosis of pre-eclampsia. Clinical studies have shown that these assays, when used together, are better predictors of pre-eclampsia than either marker alone. The addition of the assays will complement Siemens’ existing portfolio of integrated OB/GYN diagnostic solutions, which include both ultrasound imaging and reproductive endocrinology laboratory testing designed to help clinicians address the lifelong reproductive health needs of their patients.
Increasingly, healthcare professionals are looking to the integration of in vitro and in vivo diagnostics in the practice of maternal/fetal diagnostic medicine. “Nephromics is pleased to work with a leader in diagnostic medicine with a strong commitment to advancing maternal/fetal wellness,” said John Gerber, CEO, Nephromics LLC. “Together, I am confident that we will be able to make these tests available to healthcare professionals and their patients throughout the world.”
Digital Edition
Lab Asia 31.6 Dec 2024
December 2024
Chromatography Articles - Sustainable chromatography: Embracing software for greener methods Mass Spectrometry & Spectroscopy Articles - Solving industry challenges for phosphorus containi...
View all digital editions
Events
Nov 27 2024 Istanbul, Turkey
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK
Jan 25 2025 San Diego, CA, USA
Jan 27 2025 Dubai, UAE
.jpg)