Laboratory Products
Key Considerations When Purchasing Your Biological Safety Cabinet
Sep 02 2015
The purchase of a biological safety cabinet should be made with much consideration. These cabinets serve as the Primary Engineering Control, the first line of defense in providing personnel, environmental and/or product protection against biohazards and potentially harmful agents in laboratory, research, clinical and industrial settings.
Laboratory safety starts with risk assessment, followed closely with engineering controls, the primary being the biological safety cabinet, playing a key role in ensuring that infectious “microorganisms” and potentially harmful particulates and/or aerosols can be handled safety.
Perform a detailed risk assessment with a Certified Biosafety Safety Professional (CBSP) or Industrial Hygienist who has knowledge of the risk levels associated with specific biological materials and chemicals that may be used. This will determine the type of materials used in the cabinet as well as the potential risks. The risk assessment should focus on three critical areas:
- Personnel protection from harmful biological agents inside the cabinet.
- Product protection to avoid cross contamination of the work, experiment or process being performed.
- Environmental protection from contaminants that are used within the cabinet.
These areas contains specific risks, give each full consideration when developing the risk assessment. Critical to a successful risk assessment is making sure the input you receive is comprehensive from your Certified Biosafety Safety Professional (CBSP) or Industrial Hygienist.
There are different Classes of biological safety cabinets based on the level of protection they are required to provide. Within those Classes are various Types designed to address specific needs. The National Sanitation Foundation (NSF) has established performance standards that cabinets must meet to be Classified and listed as a specific Type of cabinet. The Standard NSF/ANSI 49 for Biosafety Cabinetry is reviewed every five years and it’s recommended to certify your cabinet on an annual (according to NSF/ANSI 49) to biannual basis (according to standard USP <797> for Compounding Pharmacy Products). It is required to solicit input from a qualified biosafety officer and Environmental Health and Safety (EHS) professional.
Overview of Biological Safety Cabinet Classifications:
Class I – Offers personnel and environmental protection only. Class I cabinets are suitable for work involving low to moderate risk agents where there is a need for containment, but not product protection. Learn more how Class I Biosafety Cabinets work.
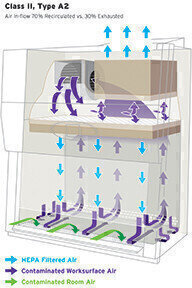
Class II – A Class II Biosafety Cabinet must meet established safety requirements for protection of product, personnel and the environment as defined by NSF/ANSI, EN12469 or another internationally recognized organization. Within the Class II classification, sub-categories have been established to define specific Types of Class II Cabinets. Learn more how Class II Biosafety Cabinets work.
Class III - The Class III cabinet was designed to work and handle highly infectious microbiological agents, unknown agents, and/or to conduct hazardous operations providing maximum protection for personnel and the environment. The Class III cabinet is completely gas tight providing access to the work zone only through an isolation area that can be routinely decontaminated between uses. Learn More how Class III Biosafety Cabinets work. Shop NuAire Biological Safety Cabinets.
To learn more visit www2.nuaire.com/06321
Digital Edition
Lab Asia 31.6 Dec 2024
December 2024
Chromatography Articles - Sustainable chromatography: Embracing software for greener methods Mass Spectrometry & Spectroscopy Articles - Solving industry challenges for phosphorus containi...
View all digital editions
Events
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK
Jan 25 2025 San Diego, CA, USA
Jan 27 2025 Dubai, UAE
Jan 29 2025 Tokyo, Japan


.jpg)