Laboratory Products
Rapid Molecular Test for Salmonella receives AFNOR Validation
Jul 01 2013
Neogen’s ANSR™ for Salmonella has been validated and approved in accordance with the current and stringent criteria of a NF Validation study carried out according to the EN ISO 16140 standard.
This new validation (NF Validation by AFNOR certification NEO 35/02-0513) follows the kit’s initial approval by AOAC International and testifies to Neogen’s dedication to increasing its line of accredited pathogen test kits for accurate and rapid results with minimal steps for users.
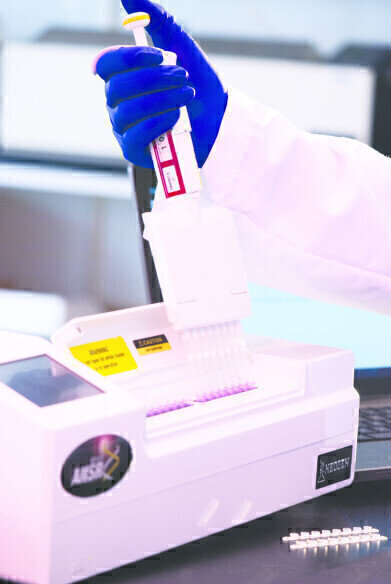
The ANSR system uses an innovative isothermal DNA amplification process and fluorescent molecular beacon technology for detection of the pathogen target.
“The NF validation of ANSR for Salmonella will allow Neogen access to a number of European markets, and beyond, where the use of the test requires an accreditation study carried out according to the AFNOR standard,” said Neogen’s Steve Chambers. “The ANSR for Salmonella system cuts the wait time for definitive results, with only 10 minutes of reaction time following sample enrichment. Other commercially available molecular amplification tests require up to 3 hours of reaction time.”
In the NF validation study, ANSR for Salmonella was found to be an effective procedure for the detection of Salmonella in meat, poultry, dairy, seafood and vegetables. Neogen is currently pursuing a similar validation of its ANSR for Listeria rapid molecular test system.
Unlike PCR-based methods, ANSR requires only a single reaction temperature, which completely eliminates the time-consuming heating and cooling cycles of older methods. The ANSR system’s small bench top footprint and extremely simple test procedure make it an easy fit in any laboratory workflow.
To learn more about the ANSR system visit www.neogeneurope.com/ANSRTestDrive.
Digital Edition
LMUK 49.7 Nov 2024
November 2024
News - Research & Events News - News & Views Articles - They’re burning the labs... Spotlight Features - Incubators, Freezers & Cooling Equipment - Pumps, Valves & Liquid Hand...
View all digital editions
Events
Nov 18 2024 Shanghai, China
Nov 20 2024 Karachi, Pakistan
Nov 27 2024 Istanbul, Turkey
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK