Laboratory Products
High-precision measurement devices for high-precision measurements of NaOH
Mar 09 2015
What do pretzels, coloured fabrics, soap, and aluminium foil have in common?
They’re all made using sodium hydroxide (NaOH, also known as lye or caustic soda). This strong base is a staple of the chemical industry, and its applications range from the food and beverage industry to petroleum, and nearly everything in between.
The production of downstream products like solvents, plastics, fabrics, adhesives, herbicides, dyes, inks and pharmaceuticals requires NaOH, too.
Take, for example, the magazine you’re reading right now. Sodium hydroxide, together with sodium sulphide, was a critical component of the ‘white liquor’ used to remove lignin from the wood pulp in the production of the paper. NaOH also played an important role during the bleaching of the pulp and even the ink and dye used to print these words was probably manufactured from NaOH as a basic chemical feedstock. When you’re done with the magazine, NaOH will be used to remove that same ink during the recycling.
Not convinced? How about the purified water you’re drinking? NaOH played a role in the chemical synthesis of the polycarbonate bottle and the acidity and heavy metal control of its contents. And when you’re finished with the bottle, a NaOH solution will be used by a recycling plant to clean it.
So what’s the issue? The above products, like many others, require the determination of NaOH concentration for product consistency. Traditionally, titration was used to determine this value, but this process is time-consuming, labour-intensive, and imprecise. Because of the tendency of NaOH to absorb both water and carbon dioxide from the air, the longer the measuring process, the more the NaOH solution will be affected. This leads to reduced accuracy of the concentration measurement. The answer? Rapid and precise measurement of NaOH concentration using refractometry.
Refractometers are used to measure the refractive index of a sample, a value that will remain constant between formulations with identical concentrations of ingredients. Although inaccurate temperature control and low inter-operator reliability pose problems for the use of Abbe-type refractometers in quality control, modern automatic Abbemat refractometers from Anton Paar eliminate these issues with accurate internal temperature control systems and automatic operation. These modern devices are capable of providing the kind of rapid, serial quality checks that are required on the assembly line.
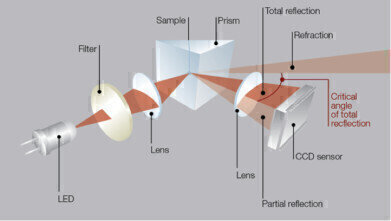
They operate by means of an LED light source, which sends a beam of single-wavelength light through a measuring prism in contact with a sample at a controlled temperature (Fig. 1). Depending on the difference of the refractive indices between the NaOH solution and prism, the light is partly refracted and reflected, or totally reflected. The critical angle of total reflection is determined by measuring the reflected light intensity on a CCD array as a function of the incident angle (Figure 1).
Figure 1. The measuring principle of an Abbemat automatic refractometer
The temperature is controlled to within 0.03 °C by an electronic temperature control, such as an internal Peltier control. This allows for measurements of refractive index with a precision of within 0.00002 nD, well above the precision possible with other methods.
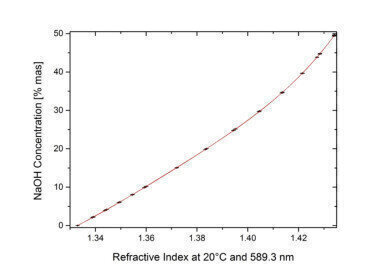
Anton Paar refractometers offer several useful features for the measurement of NaOH. Besides the internal Peltier temperature control, complete user-independence and high quality, internal optics allow the determination of the concentration of NaOH with up to 0.01 %mas accuracy (Figure 2).
Figure 2. The refractive index correlates with concentration of NaOH with an accuracy of up to ±0.01 % in the 0.0 % to 25.0 % range, and an accuracy of up to ±0.02 % in the 25.0 % to 50.0 % range for the Abbemat refractometers from Anton Paar.
Reproducibility and repeatability were tested in Anton Paar laboratories.
Specially designed accessories for NaOH measurements are also available according to the application. For simple measurement of low-concentrated solutions, a few drops can be placed directly in the optional Hastelloy B3 sample well. If automation or the measurement of many samples is required, special micro flow cells, made of perfluoroalkoxy alkanes (PFA), are ideal for the measurement because of their high material resistance. Using this setup, serial quality control checks can be performed without ever having to come into contact with the sample during measurement, which also would be recommended for high-concentration measurements.
NaOH is everywhere, and Anton Paar is there to measure it.
Digital Edition
Lab Asia 31.6 Dec 2024
December 2024
Chromatography Articles - Sustainable chromatography: Embracing software for greener methods Mass Spectrometry & Spectroscopy Articles - Solving industry challenges for phosphorus containi...
View all digital editions
Events
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK
Jan 25 2025 San Diego, CA, USA
Jan 27 2025 Dubai, UAE
Jan 29 2025 Tokyo, Japan
.jpg)