Gas Chromatography
Improving selectivity and sensitivity in pharmaceutical nitrosamine testing
Jun 10 2024
Pharmaceutical manufacturers face significant challenges in ensuring the safety and quality of their products, particularly with the detection of nitrosamines, which are potentially harmful contaminants. Detecting nitrosamines accurately and efficiently is crucial but can consume substantial time, money, and resources. Many companies outsource their testing to specialised laboratories, which can lead to lengthy turnaround times and reduced control over the testing process.
GC-MS/MS (Gas Chromatography-Tandem Mass Spectrometry) is a widely used and well-established method for detecting nitrosamines in pharmaceuticals. This technique combines gas chromatography with mass spectrometry to identify and quantify trace levels of nitrosamines. However, in complex pharmaceutical matrices, GC-MS/MS can face challenges with selectivity, making it difficult to differentiate nitrosamines from other closely eluting compounds and background contaminants. These challenges can sometimes lead to delays in product release, impacting both the company's costs and its reputation for delivering safe products on time.
Selectivity in nitrosamine testing is critical because it directly affects the accuracy and reliability of the results. In the pharmaceutical industry, ensuring that products are free from harmful contaminants like nitrosamines is essential for both regulatory compliance and patient safety. High selectivity in testing methods allows companies to precisely identify and quantify nitrosamines, even in the presence of other compounds, thereby avoiding false negatives and ensuring that no harmful levels of nitrosamines are missed.
High sensitivity in testing methods ensures the accurate detection of even trace amounts of nitrosamines, preventing false alarms and missed detections. This reliability is crucial for maintaining consistent product quality, meeting regulatory standards, and avoiding costly delays and additional testing. Therefore, a testing method with superior selectivity, such as Ellutia’s GC-TEA (Gas Chromatography-Thermal Energy Analyser), is essential for optimising production efficiency, ensuring product safety, and maintaining regulatory compliance.
The operational costs associated with GC-MS/MS systems can be substantial, requiring significant investments in purchase, maintenance, and operator training. The extensive sample preparation required for GC-MS/MS further increases costs and extends turnaround times, straining financial resources and potentially leading to higher product prices or cuts in other areas. This complexity also reduces the reproducibility of results and increases the likelihood of errors, impacting the reliability of the quality control process and slowing down production timelines.
GC-TEA offers a cost-effective alternative with simpler operation, reduced maintenance needs, and minimal background interference. By streamlining sample preparation, typically involving methanol extraction, centrifugation, and filtration, GC-TEA enhances reproducibility and reliability, enabling pharmaceutical companies to maintain consistent quality standards more effectively. This efficiency allows companies to allocate resources more efficiently and invest in critical areas such as innovative research and development, ultimately enhancing their competitive edge. Furthermore, the GC-TEA is designed to interface with most GC systems, making integration into existing laboratory setups straightforward and efficient.
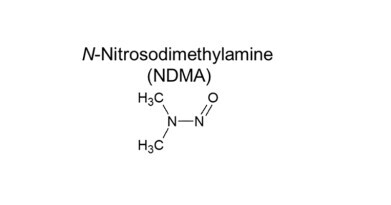
In practical applications, the GC-TEA has shown to be effective in detecting nitrosamines, such as NDMA, in various products like Metformin, Ranitidine, and Sartan tablets. GC-TEA’s capability to analyse different sample sizes and identify nitrosamine distribution variability within tablets makes it a versatile and reliable option. This helps ensure compliance with safety standards and improves overall quality control processes.
Pharmaceutical companies using GC-MS/MS for nitrosamine detection face challenges related to method development, sensitivity, operational costs, and sample preparation complexity. While GC-MS/MS is a valuable tool with proven effectiveness, GC-TEA offers solutions to these specific challenges by providing higher selectivity, minimal background interference, lower operational costs, and simpler sample preparation. Integrating GC-TEA into testing protocols can enhance the reliability and efficiency of nitrosamine detection, ensuring the safety and quality of pharmaceutical products and helping companies meet regulatory requirements more effectively.
More information online
Digital Edition
Lab Asia 31.6 Dec 2024
December 2024
Chromatography Articles - Sustainable chromatography: Embracing software for greener methods Mass Spectrometry & Spectroscopy Articles - Solving industry challenges for phosphorus containi...
View all digital editions
Events
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK
Jan 25 2025 San Diego, CA, USA
Jan 27 2025 Dubai, UAE
Jan 29 2025 Tokyo, Japan