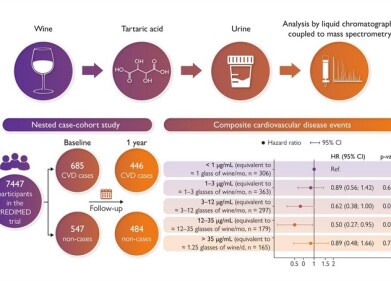
Chromatography
From Discovery to Production of Active Biotherapeutic and Pharmaceutical Ingredients
Jun 19 2018
Kromasil fits across the entire biotherapeutic and pharmaceutical organisations. Typically, researchers carry out method development using Kromasil UHPLC and HPLC columns based on 1.8 - 5 μm particle sizes. Then scientists and engineers in discovery, process and manufacturing departments scale the method up according to their needs using the same Kromasil stationary phases in prepacked columns and bulk ranging from 7 to 25 μm. This Kromasil capability to supply the entire development and production path of an API is unique in the market and shortens the timeline plus minimises costs when producing a drug. With 30 years of experience, Kromasil continues to expand its list of products, provides ready-made columns as well as bulk material for normal phase, reversed phase, chiral, HILIC, SFC and SMB chromatography.
For more information go to www.kromasil.com.
Digital Edition
Lab Asia 31.6 Dec 2024
December 2024
Chromatography Articles - Sustainable chromatography: Embracing software for greener methods Mass Spectrometry & Spectroscopy Articles - Solving industry challenges for phosphorus containi...
View all digital editions
Events
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK
Jan 25 2025 San Diego, CA, USA
Jan 27 2025 Dubai, UAE
Jan 29 2025 Tokyo, Japan