Chromatography
Screening for Nitrosamines in Pharmaceutical Products
Feb 24 2020
Nitrosamine are carcinogenic compounds so need to be monitored to ensure unsafe levels are not found in consumer products. Due to unsafe levels being found in a number of pharmaceutical products the EMA (European Medicines Agency) is requiring pharmaceutical companies to complete and submit a risk assessment by 26th March. This means many pharmaceutical manufacturers will potentially need to screen their products for nitrosamine content.
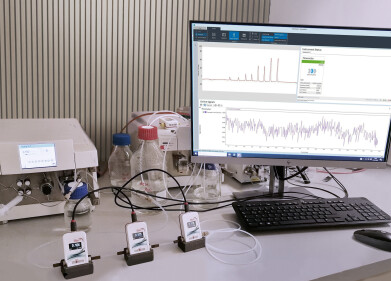
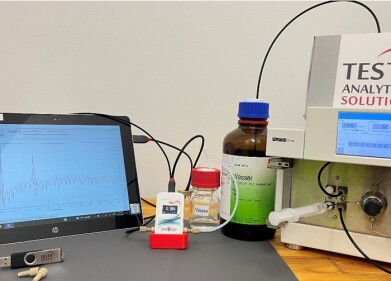
The Ellutia 800 Series TEA is perfectly suited to nitrosamine detection thanks to is selectivity and sensitivity for Nitroso compounds. For testing of pharmaceutical drugs, The 800 Series TEA can be interfaced to a chemical stripping system that allows for rapid testing of ATNC (Apparent Total N-nitrosamine content). This quickly gives an accurate result for the total nitrosamine content of a sample showing both volatile and non-volatile components.
Any positive sample can then be further analysed by an 800 Series TEA interfaced to a GC where volatile nitrosamines such as NDMA (N-Nitrosodimethylamine) can be separated and quantified.
More information online
Digital Edition
Lab Asia 31.6 Dec 2024
December 2024
Chromatography Articles - Sustainable chromatography: Embracing software for greener methods Mass Spectrometry & Spectroscopy Articles - Solving industry challenges for phosphorus containi...
View all digital editions
Events
Jan 22 2025 Tokyo, Japan
Jan 22 2025 Birmingham, UK
Jan 25 2025 San Diego, CA, USA
Jan 27 2025 Dubai, UAE
Jan 29 2025 Tokyo, Japan